The determination and interpretation of biological structures at the atomic level is an essential part of biopharmaceutical industry and life science R&D, with applications in:
Our holistic approach to structure determination allows us to choose the best method for any target, from single small proteins to complex intercellular arrangements.


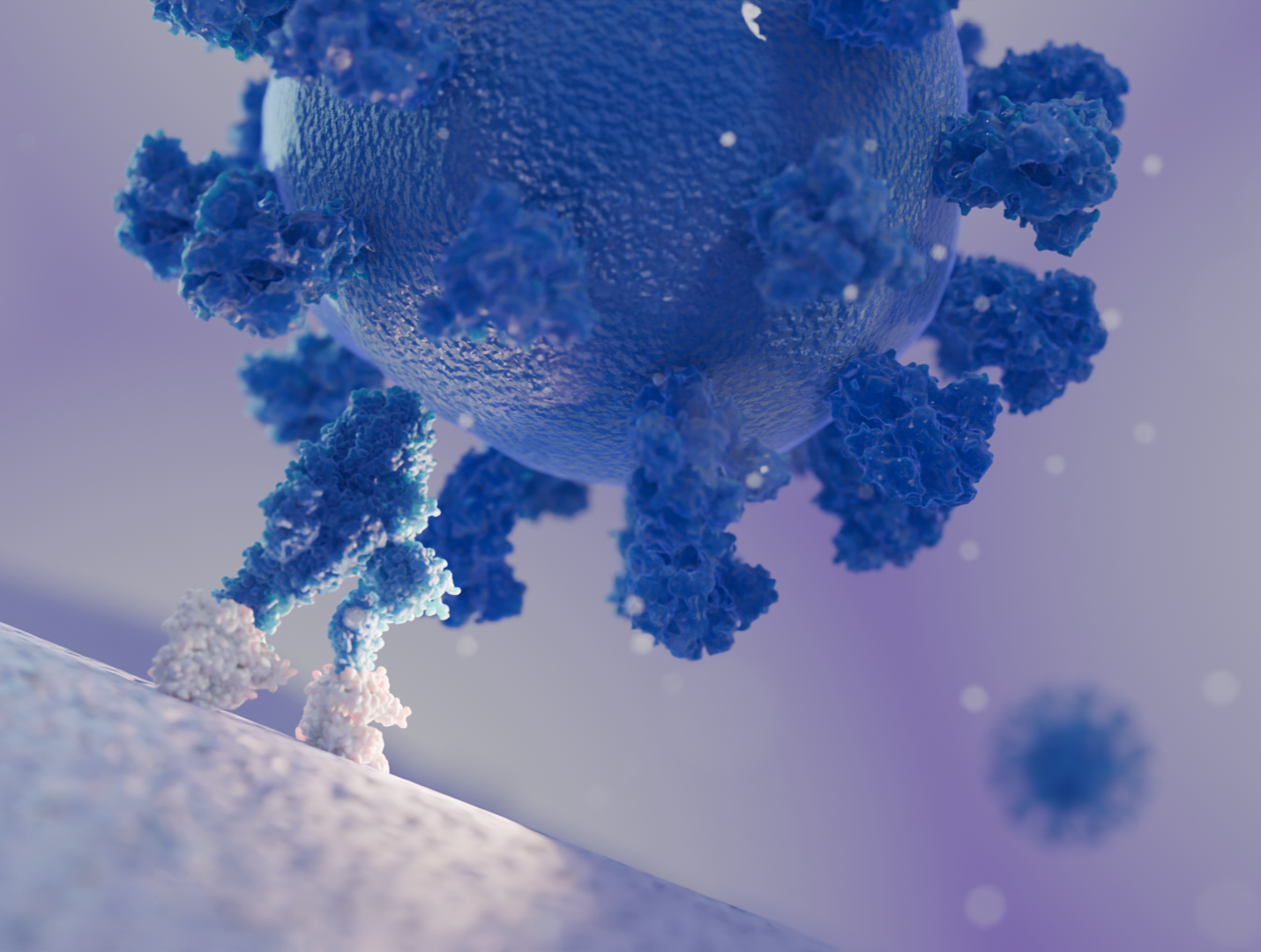
Learn more about the newest advances in host-pathogen research by Cryo-ET here.
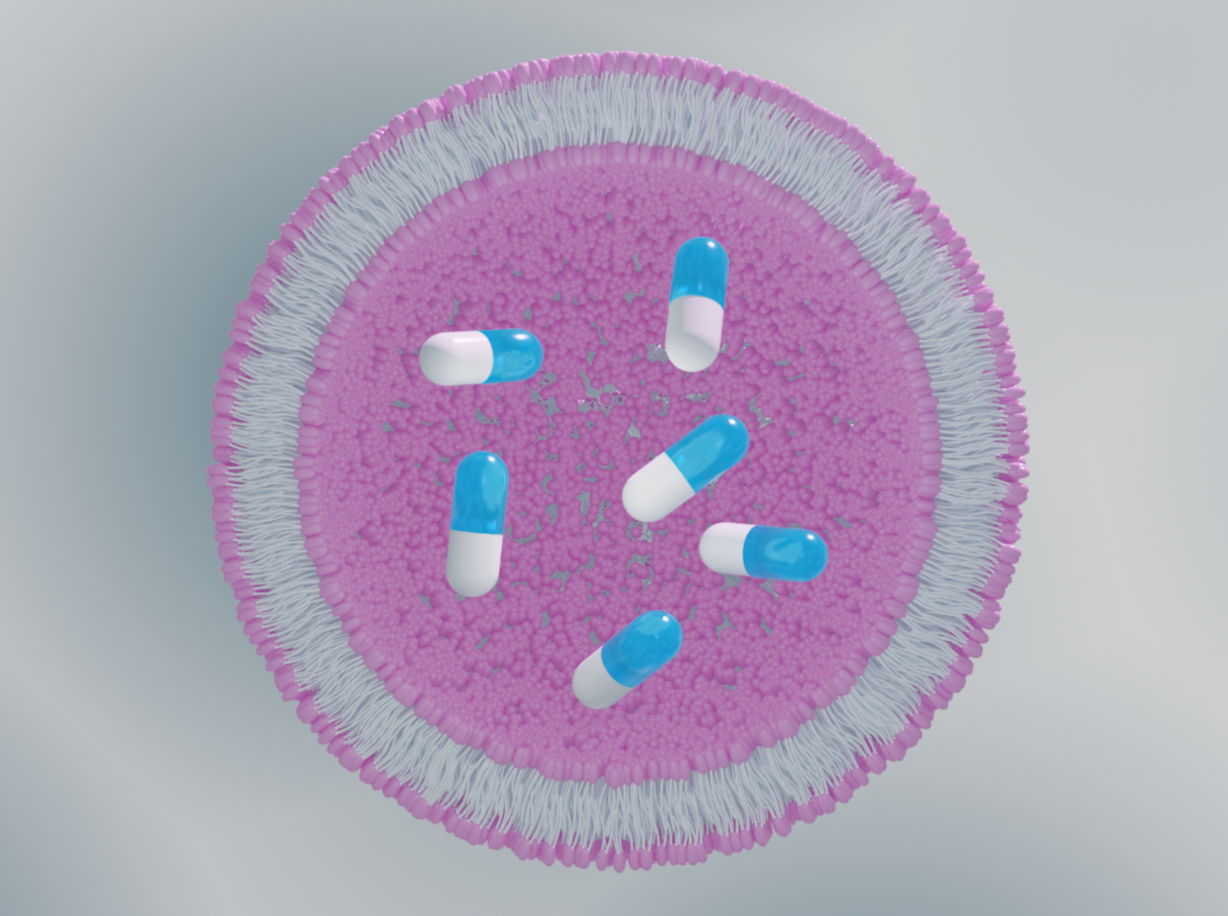
Structural biology approaches grant knowledge of the vector’s loading state, morphology, and distribution, which are all key to successful drug delivery vector development.

However, predictions remain only that until validated with experimental methods. Structural biology experiments ensure the accuracy and applicability of AI-predicted proteins in real-world scenarios, which is especially important in the area of Biopharmaceuticals.

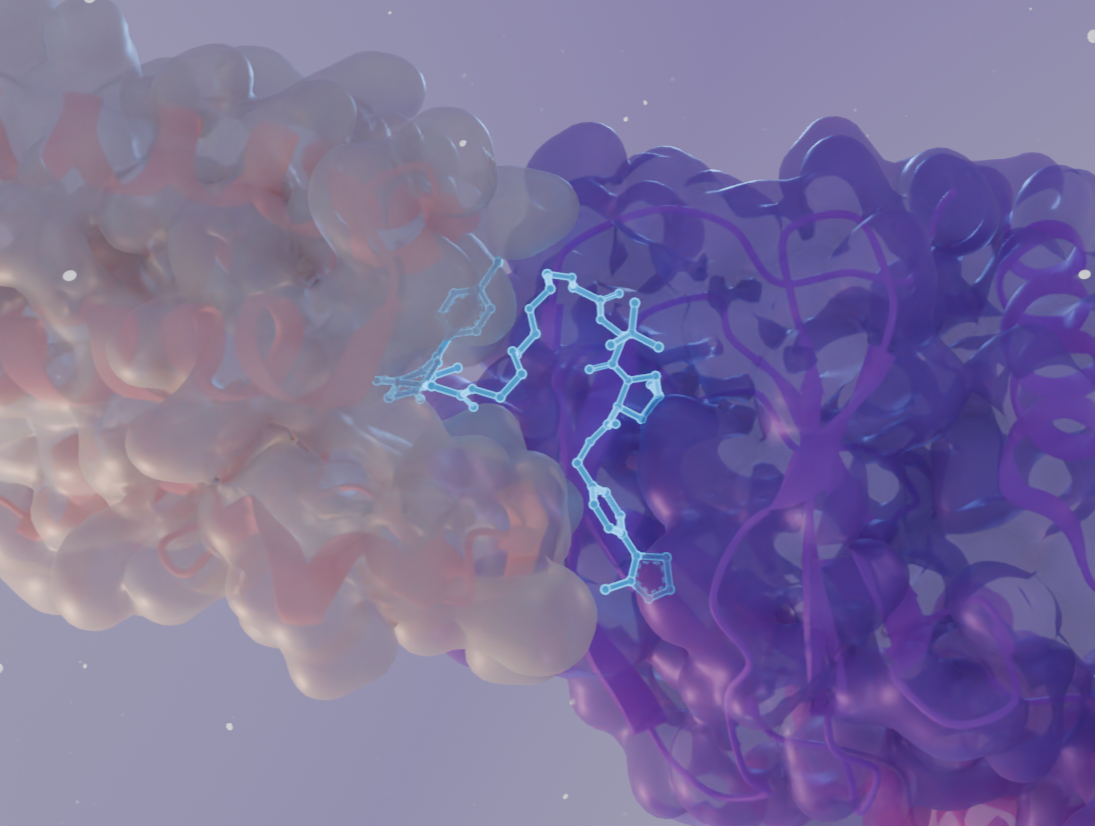
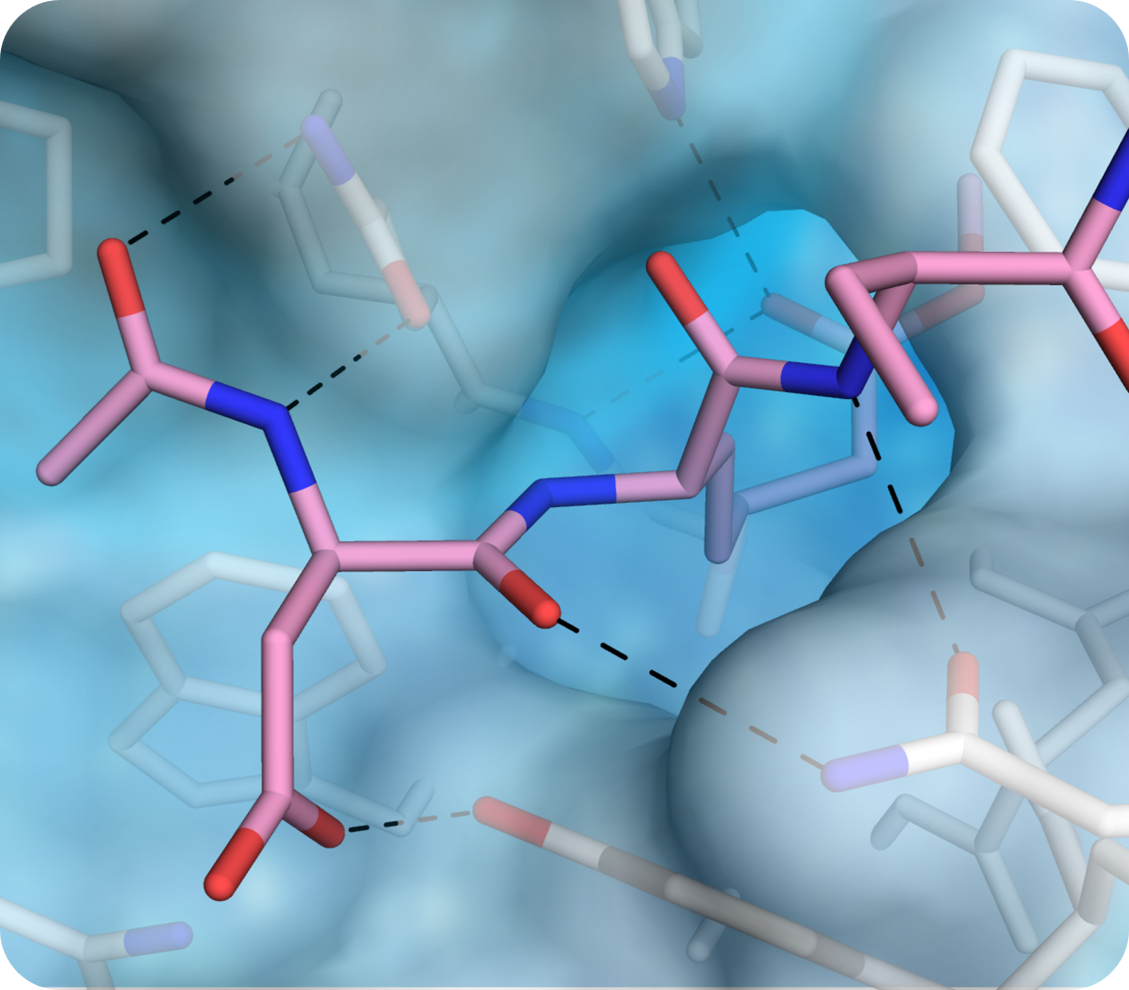
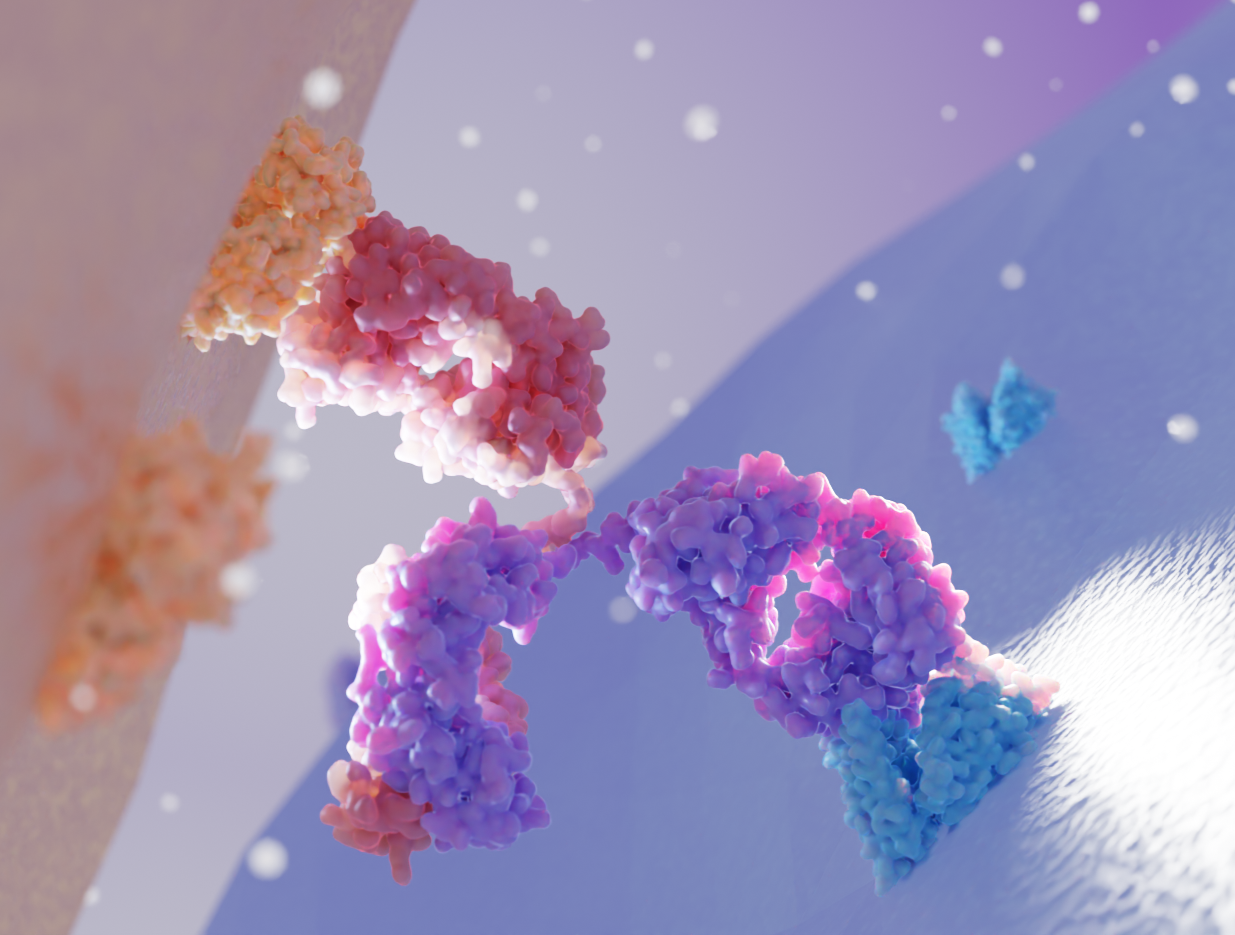
By providing high-resolution insights into protein-protein interactions, ligand binding, and complex assembly, structural biology enables the rational design of these molecules.
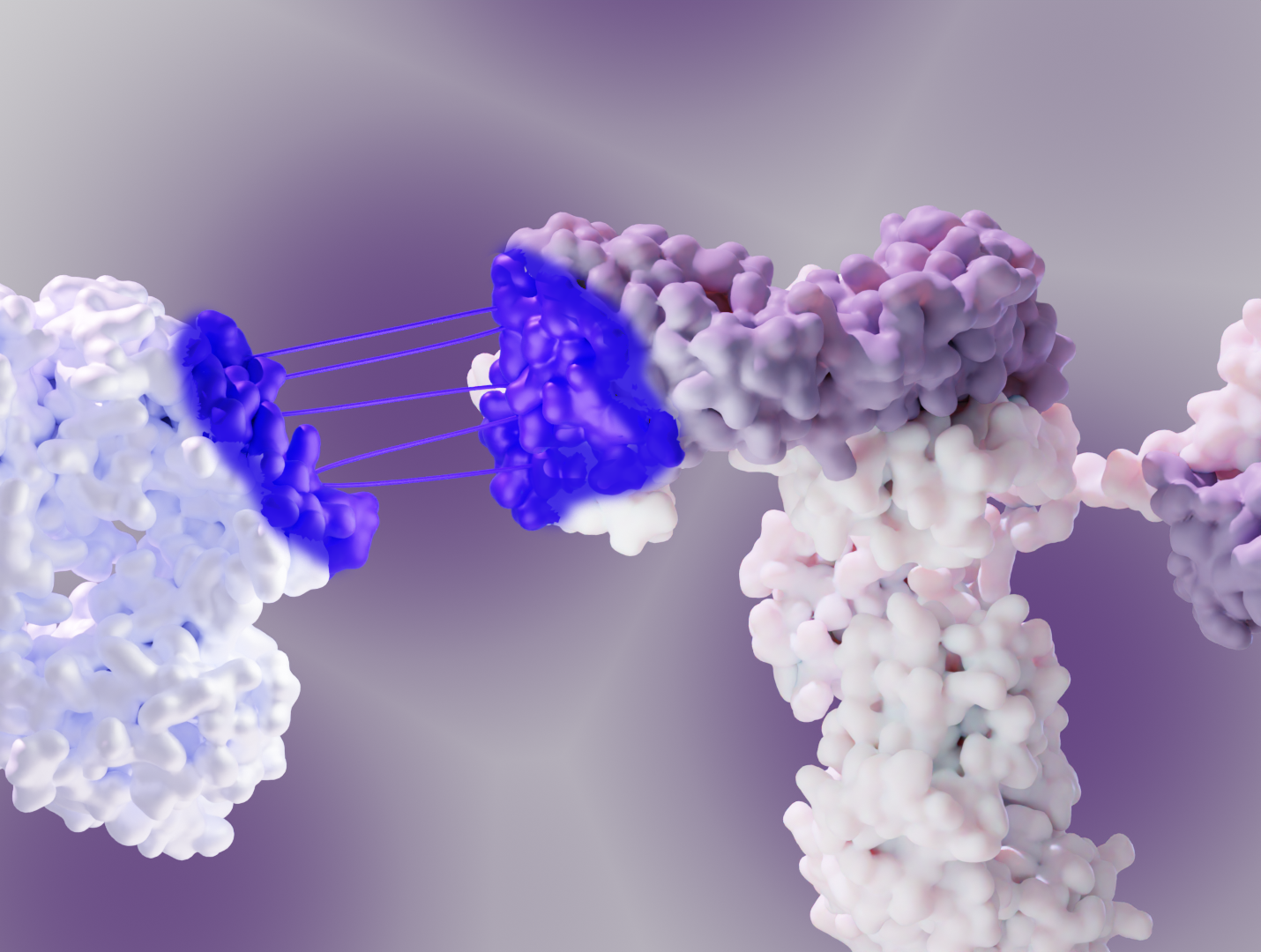
X-ray crystallography and cryo-EM help visualize the ternary complexes formed between the target protein, the E3 ubiquitin ligase, and the PROTAC molecule. This allows researchers to optimize the linker length and orientation for efficient ubiquitination and subsequent degradation.