In the midst of summer warmth, it might seem odd to think about seasonal infections. Yet we know that not so far from now, autumn will bring a sharp rise in influenza cases, even among the vaccinated. Despite decades of research and ongoing vaccination efforts, the influenza virus remains a serious global health threat, contributing to up to 650,000 deaths worldwide each year.
Our current defenses, including vaccines and antivirals, often target the virus’s rapidly mutating envelope proteins. This high mutation rate leads to decreased drug efficacy and the emergence of drug resistance over time. A need for anti-influenza strategies targeting more evolutionarily conserved parts of the virus have been unsuccessful, largely due to lack of structural information about the components – until now.
The elusive RNP: a major structural biology challenge
Recent research published in Science offers a promising new direction by looking at the influenza virus’s operational core: the ribonucleoprotein (RNP) complex. Unlike the surface proteins, the RNP is evolutionarily conserved, making it a much better target for developing therapies that could work against many different flu types and for a longer time. Each RNP complex is comprised of a viral genomic RNA segment, multiple nucleoprotein (NP) subunits, and a polymerase complex (FluPol) responsible for transcription and replication of the viral genome within the host cell. Without a correctly functioning RNP, the virus can’t reproduce and is therefore deactivated.
Despite its critical role, gaining a high-resolution structural understanding of the RNP assembly and its working mechanism has remained elusive for decades. The primary obstacle has been the RNP’s inherent flexibility and dynamic nature, making it incredibly difficult to capture with traditional methods like single-particle analysis (SPA) Cryo-EM or X-ray crystallography. While individual pieces of the puzzle could be imaged (including NP subunits and FluPol), combining the information together to understand the whole context of RNP assembly and mechanism couldn’t be achieved.
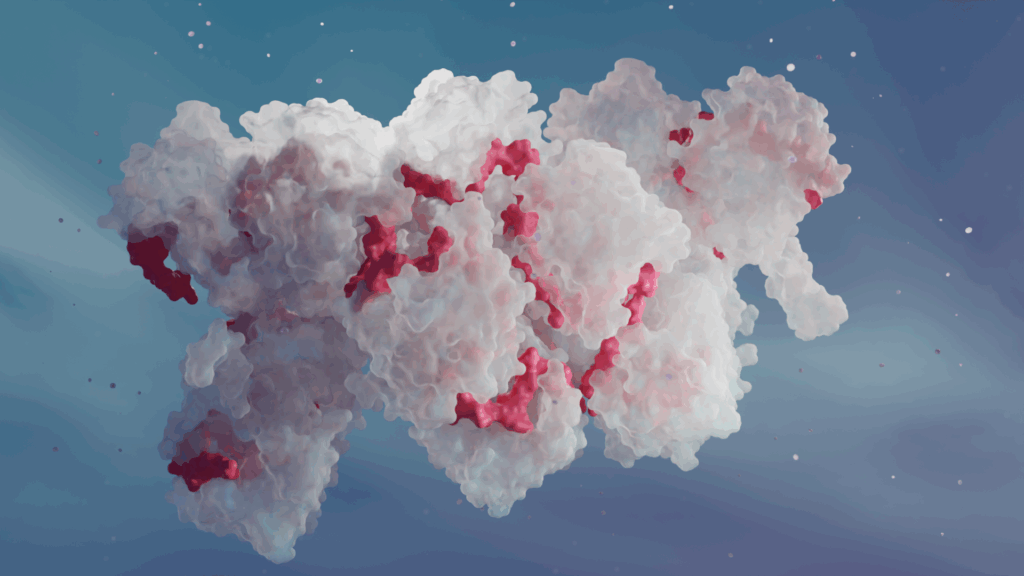
Cryo-ET: see the previously unseen
This new study from University of Pennsylvania combined cutting-edge cryo-EM techniques: SPA and cryo-electron tomography (Cryo-ET) – and it was Cryo-ET that finally allowed scientists to see the larger RNP assembly (see image above).
Cryo-ET allows researchers to look at individual cell components, such as proteins or protein complexes, directly in the cell. In this case, Cryo-ET enabled the visualization of the RNP directly in the cell and in distinct functional states, capturing its natural variability and, most importantly, revealing how FluPol interacts with RNP in situ.
To overcome the RNP’s persistent flexibility, the researchers employed clever strategies: For SPA, they reconstituted the shortest possible RNP part: the nonstructural (NS) segment of the influenza virus, which is naturally less flexible than the whole RNP. And to see the RNP and polymerase using Cryo-ET, they used special grids coated with graphene oxide, which helped stabilize the delicate samples and preserve the polymerase density during the imaging process.
The intricate replication mechanism of the influenza virus
Insights from Cryo-ET revealed the overall architecture of the influenza RNP: it’s not a single helix like in many other viruses, but a right-handed, antiparallel double helix of NP polymers, with the viral RNA securely placed within its minor groove. This immediately raised a question of how can the FluPol access the RNA template if it’s buried inside the double helix and the polymerase is on the outside.
The question was answered by Cryo-ET visualizations of the preinitiation and elongation FluPol complexes: as the polymerase processes along the RNA template for RNA synthesis, the overall double-helical architecture of the RNP is maintained. But the NP subunits can temporarily change their conformation to release their grip on the RNA, allowing the polymerase to process that section. Once the polymerase passes, the NP subunits snap back into their original conformation, resecuring the RNA.
This mechanism, even though energetically costly, provides significant advantages to the virus: it likely enhances processivity (helping the polymerase make longer RNA copies more efficiently) and helps the virus evade host immune detection. By keeping its RNA largely hidden only exposing small parts for a short time, the virus minimizes the chance of triggering cellular alarms that detect viral RNA.
New avenues for antiviral drug development
Beyond fundamental understanding, these Cryo-ET insights can directly inform antiviral development. The study pinpointed a promising new target: the binding interface that connects adjacent NP subunits and holds the RNP helix together. This interface is remarkably conserved across different influenza A and B subtypes, providing a potential target for broad-spectrum drug design. The researchers validated the essential role of this interface by showing that disrupting it abolished RNP assembly. Leveraging the detailed structural information, they then designed pharmacophore models (3D chemical search templates) and performed extensive virtual screening of over 30 million chemical structures. This approach led to the identification of several lead compounds that effectively inhibited influenza virus replication in cell-based assays.
In conclusion, this study demonstrated how the advancements in Cryo-ET are transforming the structural biology landscape, allowing researchers to tackle previously intractable problems like the highly flexible influenza RNP. We are looking forward to see how this study will contribute to the development of next-generation, broad-spectrum antiviral therapeutics targeting the conserved RNP!
Thanks to the authors: Ruchao Peng and colleagues for producing this great work! You can read the paper here (unfortunately, behind a paywall).
If you want to find out more about Cryo-ET and whether it could advance your own research and development pipelines, contact us here or send an email to hello@bimovis.com. We will happily schedule a free, non-binding meeting!